Abstract
The Belgian sickle cell disease registry (BCR) was initiated in 2008 and aims to evaluate mortality, morbidity as well as clinicals practices in participating centers. The current analysis focuses on criteria influencing age at transplantation (HSCT) and on the management of Hydroxyurea (HU) therapy across centers.
The methodology of the registry has already been published (Le PQ et al., Pediatric Blood and Cancer, 2015) . Data are recorded prospectively from neonatal screening or first contact until last annual follow-up (FU) or death. The data collected included diagnosis, demography, treatment and outcome data as well as a minimal set of biological values. Data were extracted from the database in May 2021.
There are 1029 patients registered by 14 different centers (2 centers exclusively treating adult patients). The median FU is 9 y (1-53 y). Median age at last FU is 13 y (0-61 y). 890 patients (86,5%) have a severe phenotype (SS or Sβ°) and 52% are female. Among them, 561 (55%) are born in Belgium of whom 379 (68%) are diagnosed by neonatal screening. In the absence of neonatal screening, median age at diagnosis is 1 year (range 0-18). 131 patients have been transplanted (126 successfully), 68 HSCT were performed before 2005. At last FU, 646 patients (76%) received at least 1 disease-modifying treatment (DMT) : 598 patients receive HU, 65 are chronically transfused, 8 participate in a study with crizanlizumab. The prescribed HU dose is known for 572 patients. 179 patients (31.3%) receive less than 20 mg/kg/day, 217 (37.9%) less than 25 mg/kg/day, 148 (25.9%) less than 30 mg/kg/day and 28 (4.9%) were prescribed more than 30 mg/kg/day.
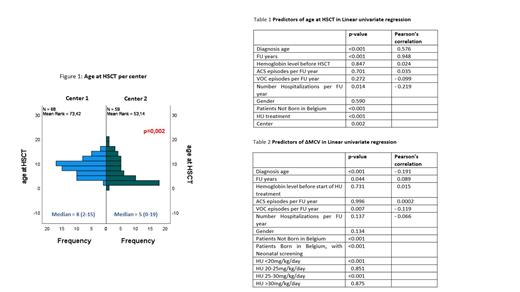
The majority of HSCT were performed in two centers (68 and 59, respectively). Median age at HSCT was significantly different between both centers (8y (2-15) versus 5y (0-19); p=0.002) (figure 1).
Variables associated with a lower age at HSCT are detailed in table 1.
In a linear multivariate regression model, birth in Belgium (p=0.002), no treatment with HU (p=0.009) and shorter duration of FU (p<0.001) but not the center are independent factors correlated with younger age at transplantation. This linear model explains 90.60% of the variance (adjusted R²) of age at HSCT.
Among not transplanted patients, the proportion of those receiving HU is different between centers (50% to 91%; p=0.050). The median age at which HU was initiated was also significantly different between centers (4y to 21y; p<0.001) as was the management of HU treatment in a multiple comparison model measured by ΔMCV (difference in MCV before start of HU versus at last FU). In a linear univariate regression model, other variables are significant predictors for the variance of ΔMCV (table 2). In the linear multivariate regression model, the variance of ΔMCV between centers is controlled by the duration of FU (p<0.001), neonatal screening (p=0.046), HU dose between 25-30mg/kg/day (p<0.001), all resulting in a higher ΔMCV, while patients not born in Belgium (p=0.033) have a lower ΔMCV. Age at diagnosis, severity of the disease (assessed by the number of VOC/FU year) and HU dose <20 mg/kg/day are not correlated with the variance in ΔMCV.
Twenty-seven (2.6%) patients died which accounted for a mortality rate of 0.24/100 patients-years (PY) which increases significantly with age (0.18/100PY <18 years, 0.35/100PY 18-40 years and 1.43/100PY >40 years; p=0.001).
Conclusions:
BSR has an excellent registration activity from participating centers and represents a reliable tool to evaluate the Belgian SCD population. Mortality remains low with a significant trend to increase with age.
Regarding treatment practices, the age at start of HU is significantly different between centers as the approach to further HU treatment, evaluated by ΔMCV. A higher dose of HU resulted in a higher ΔMCV. However, the policy to increase HU to maximal tolerated dose seems not implemented in most centers, as 2/3 of the patients are prescribed less than 25 mg/kg/day.
Being born in Belgium and no treatment with HU are associated with younger age at HSCT. Nevertheless since 2005, almost all patients were treated with HU prior HSCT, reflecting the wider implementation of HU in SCD patients living in Belgium.
Benghiat: Novartis: Consultancy; BMS: Consultancy. Labarque: Bayer: Consultancy; Sobi: Consultancy; NovoNordisk: Consultancy; Octapharma: Consultancy; Novartis: Consultancy.